
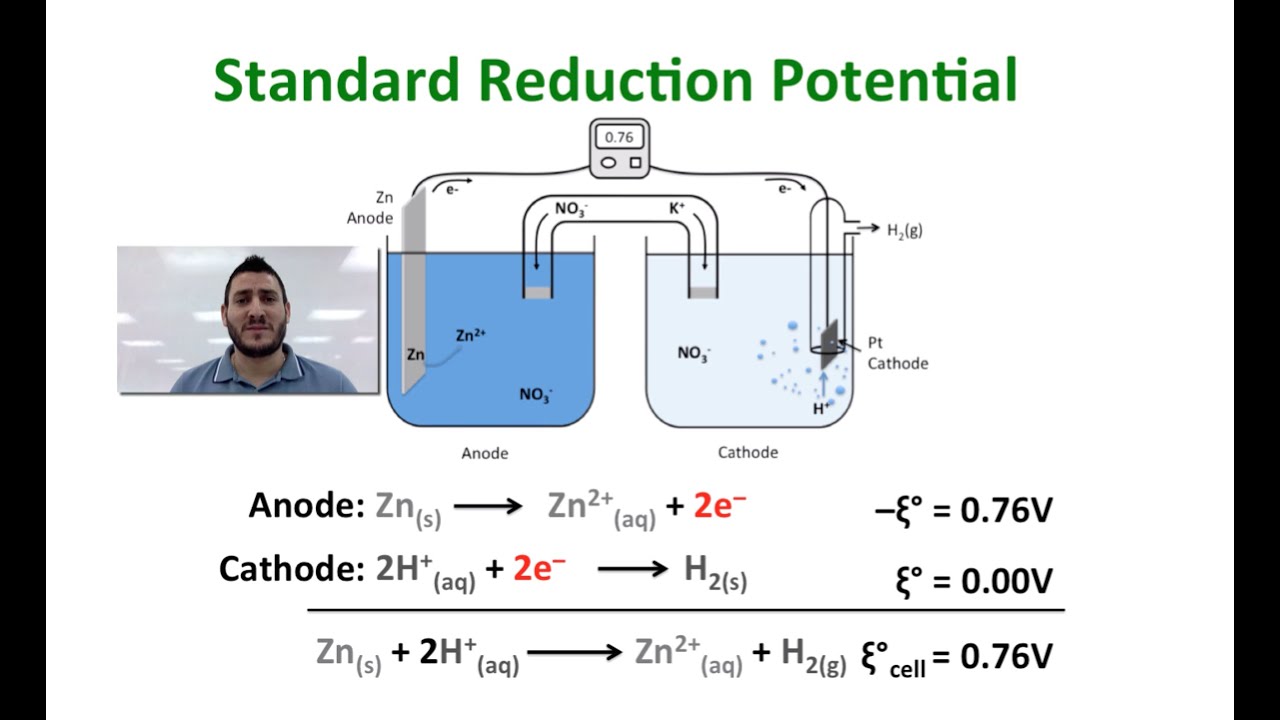
Selected Standard Reduction Potentials at 25 ☌ Half-Reactionį 2 ( g ) + 2e − ⟶ 2F − ( a q ) F 2 ( g ) + 2e − ⟶ 2F − ( a q ) A cell potential of +0.337 V is measured, and so These connections insure that the sign of the measured potential will be consistent with the sign conventions of electrochemistry per the various definitions discussed above. Since the Cu half-cell is designated as the cathode in the definition of cell potential, it is connected to the red (positive) input of the voltmeter, while the designated SHE anode is connected to the black (negative) input. A voltmeter in the external circuit allows measurement of the potential difference between the two half-cells. This approach to measuring electrode potentials is illustrated in Figure 17.6, which depicts a cell comprised of an SHE connected to a copper(II)/copper(0) half-cell under standard-state conditions.
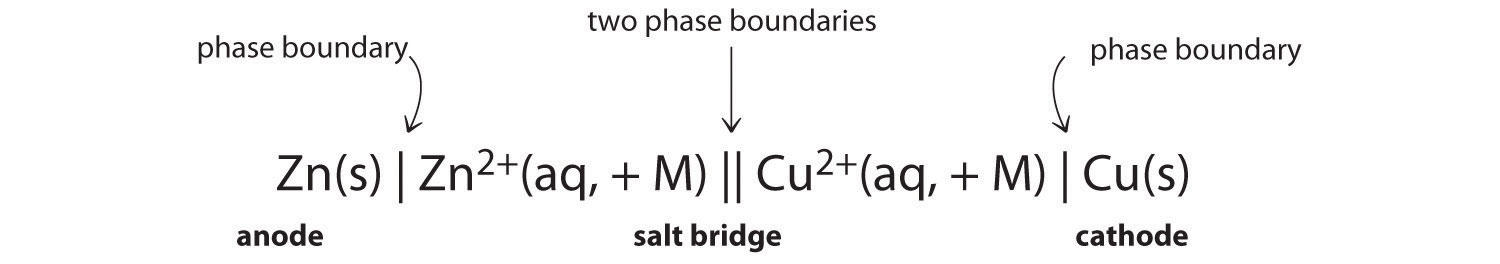
Since the definition of cell potential requires the half-cells function as cathodes, these potentials are sometimes called standard reduction potentials. When the half-cell X is under standard-state conditions, its potential is the standard electrode potential, E° X. It is only the difference in potential between two half-cells that may be measured, and these measured potentials are called cell potentials, E cell, defined asĮ cell = E X − E SHE E SHE = 0 V (defined) E cell = E X E cell = E X − E SHE E SHE = 0 V (defined) E cell = E X Instead, a half-cell potential may only be assessed relative to that of another half-cell. Considering the nature of potential in this context, it is clear that the potential of a single half-cell or a single electrode can’t be measured “transfer” of electrons requires both a donor and recipient, in this case a reductant and an oxidant, respectively. When measured for purposes of electrochemistry, a potential reflects the driving force for a specific type of charge transfer process, namely, the transfer of electrons between redox reactants. Potentials are measured in the volt unit, defined as one joule of energy per one coulomb of charge, V = J/C. This property is more commonly called voltage when referenced in regard to electrical applications, and it is a measure of energy accompanying the transfer of charge. Electrochemical cells permit this relative redox activity to be quantified by an easily measured property, potential. The two species, Ag + (aq) and Pb 2+ (aq), thus show a distinct difference in their redox activity towards copper: the silver ion spontaneously oxidized copper, but the lead ion did not. Unlike the spontaneous oxidation of copper by aqueous silver(I) ions described in section 17.2, immersing a copper wire in an aqueous solution of lead(II) ions yields no reaction. Calculate cell potentials and predict redox spontaneity using standard electrode potentials.Interpret electrode potentials in terms of relative oxidant and reductant strengths.Describe and relate the definitions of electrode and cell potentials.
By the end of this section, you will be able to:


 0 kommentar(er)
0 kommentar(er)
